Zug, Feb 20, 2023: Framesoft Document Assembly (FDA)
Framesoft announces the release of an updated version of Framesoft Document Assembly (FDA) in April 2023.
Document assembly is one of the critical components of the document automation. Framesoft’s unique Document Assembly (FDA) module includes template & component design, data integration, automatic clause assembly, template, component / clause preview and document generation as part of a workflow (e.g., a contract request workflow) or manually via action.
FDA will dramatically speed up the document template creation process based on a flexible online configuration of any “Master” document and its individual components such as e.g., sub-documents, sections, and clauses. Any component needs to be created once in the Document Assembly module and can then be used in multiple contracts templates. Each update of a document template element will lead to the creation of a new version. All former versions are still available for audit purposes via audit trail.
Conduct a review or contract negotiation of such automatically generated Contract Draft Documents. FDA will take care of:
- “Merge Handling“ of updates pushed into a currently reviewed contract via new standard clause / non-standard versions, data updates or individual updates
- Automated generation of new agreements / amendments triggered by a library clause update
- Automated generation of new agreement / amendment triggered by a contract data point update
For contract lifecycle management an extended Template Repository Search is offered, including
- Template Repository & signed contract search
- Clause search and its versions / usage in contracts
- Search for documents containing selected template components
- What-if-Analysis
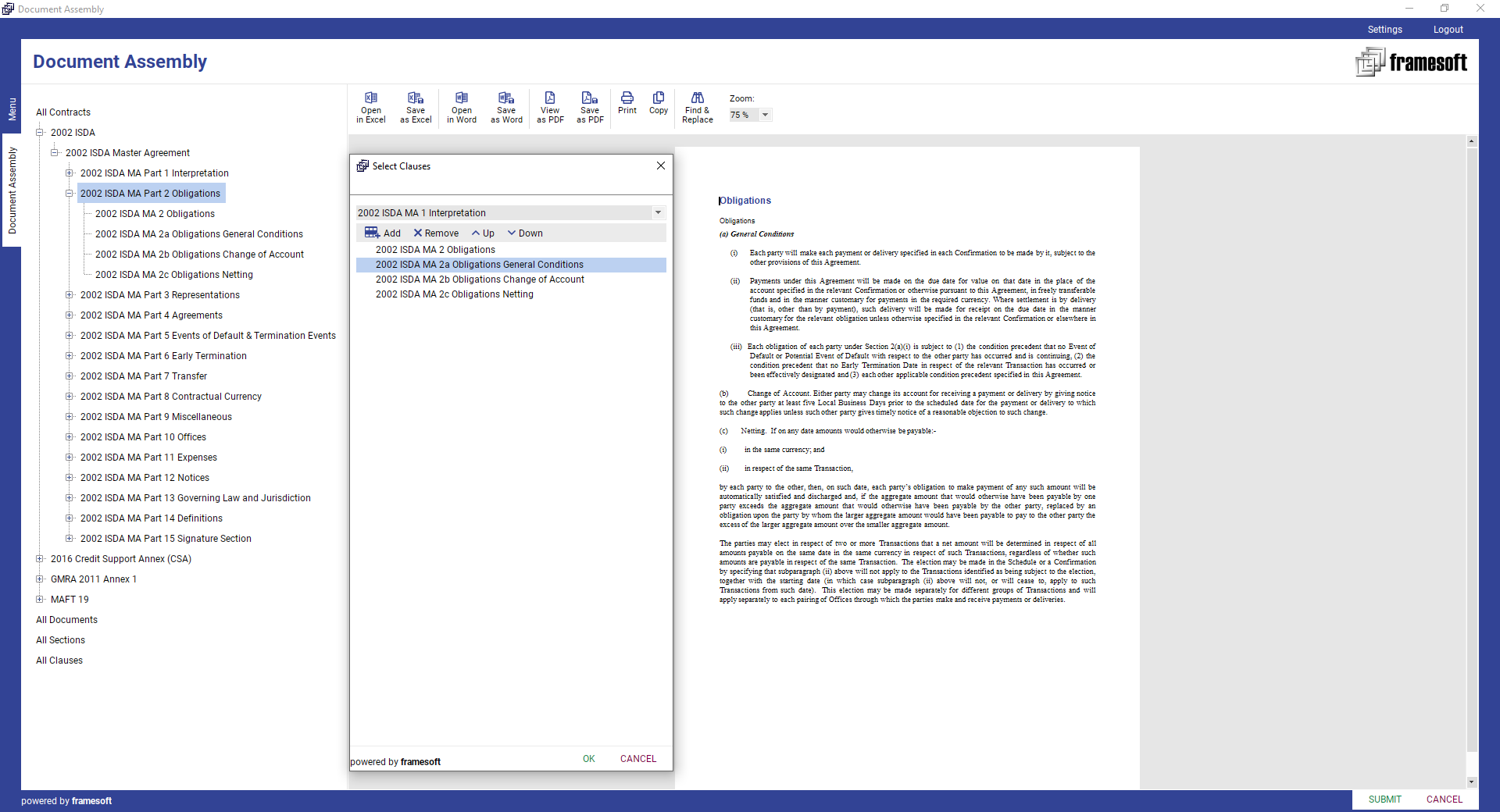
Framesoft Document Assembly (FDA) is offered as Add-On to any of our solutions.
For more information, please contact Framesoft at
