Overview
Framesoft Document Assembly (FDA) is nothing short of a revolutionary tool that is transforming the way organizations create and manage documents.
Using FDA is a game-changing innovation of the document automation strategy via combining automation, data integration, pre-designed template clauses and rules to generate new, tailor-made documents with astonishing speed and accuracy. But the thrills do not end with the document generation. Via workflow automation documents generated are provided for review, approval, e-signatures, and distribution making the process efficient from start to finish.
Automation has been in the focus of many institutions, but truly little has been done to effectively target documentation. It is not only about cost and inefficiency, but also about extremely valuable data not being used.
FDA Components
1. Clause Library
These are not just any clauses; they are often intricate blueprints designed to ensure that each document created conforms to specific stylistic guidelines, legal requirements, and branding elements. Clauses feature placeholders for variable content, making them dynamic and adaptable.
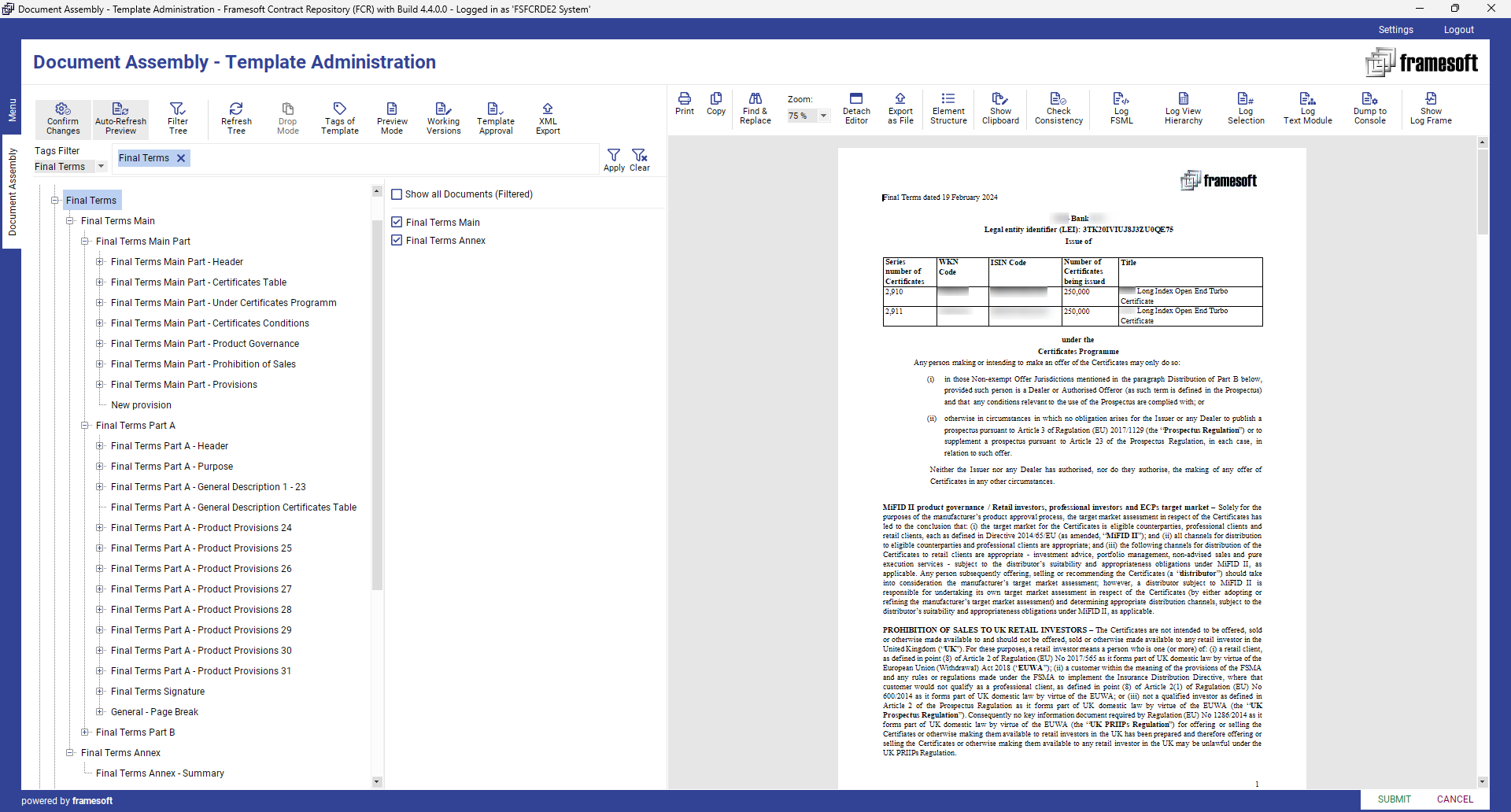
2. Document Template Assembly
By using Framesoft Document Assembly (FDA) clauses can be assembled to new or existing sections, sub-documents, and document templates on the fly. Upon its creation or update any element will be part of an approval process before being released for automated document generation.

3. Database Integration
Framesoft Document Assembly (FDA) can pull information from existing databases to populate fields and / or apply conditional logic.
4. Conditional Logic
This is a set of rules and guidelines that dictate how data is populated into the clause. For example, certain paragraphs or clauses may only appear under specific conditions.
5. Document Generation
Document Templates designed in FDA can be directly used for a document generation as follows:
- Generation integrated in Document Request Workflow
- (Re-) Generation of a manually selected document
- Document generation triggered via API call
- Further document generation actions defined
The corresponding FDA document templates are selected automatically based on an FDA mapping applied (e.g., Product, Agreement Type, Form of Organization & Jurisdiction, place of issuance or any other mapping criteria).
6. Document Post Generation Editing
When a document is generated based on FDA configuration it is presented in the document editor (Framesoft Docs).
Editing of a generated document is supported. Any change of a clause will automatically create a new “revision number” which is tracked in addition to the template clause version. This allows tracking the
- template clause versions used in the document generation
- document clauses edited upon generation
7. Version Control & Lifecycle Management
Features that track changes, maintain multiple versions, and manage the lifecycle of clauses, templates and documents are included such as e.g.,:
- View all clause & template / document version
- Generated document search
- Clause (version) usage search
- What if analysis – which generated documents would be affected by a potential clause update.
8. Workflow Automation
Advanced workflow functionalities that route the assembled & generated document for review, approval, e-signature, and distribution is a core component of FDA.
9. Output Formats
Once assembled, documents can be produced in various formats such as PDF, MS Word, or HTML, depending on the requirements.
10. Document Negotiation
Conduct document (contract) reviews & negotiations based on generated documents directly via Framesoft Online Negotiation (FON) and being supported in:
- Document negotiation with multiple (internal and external) parties
- Negotiate multiple documents in a single negotiation
- Simultaneous document revisions by the different parties involved
- Merge Handling - updates directly pushed into a negotiated document in case of new clause versions, data points or individual updates of clauses.
- Automated document generation triggered by clause or data point updates
- Messaging component for direct communication (chat) between the different parties involved
11. Document Archiving
All generated & related documents from drafts to execution copies shall be stored in a document archive system such as Framesoft Document Management (FDM).
12. Security & Compliance
Security measures such as encryption, access controls, and audit trails to ensure that sensitive data is protected and compliance with relevant regulations is assured are applied.
Framesoft Document Assembly (FDA) is seamlessly integrated into any Framesoft solution:
- Framesoft Document Generator (FDG)
- Framesoft Contract Repository (FCR)
- Framesoft Structured Products (FSP)
- Framesoft Confirmation Generator (FCG)
Contact us at
